| Time duration | May 2015 to Dec 2015 |
| Location | |
| Organization | Universiti Teknologi PETRONAS (UTP) Perak, Malaysia |
| Project | Methanol Production via CO2 Hydrogenation on Promoted Cu-based Catalyst |
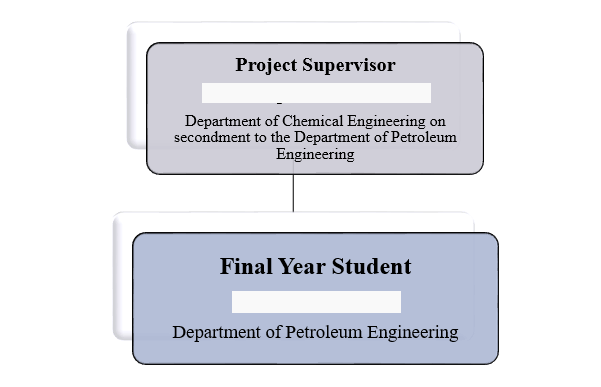
| Position | Engineering Student |
| Project Supervisor | |
| Project submitted to |
This career episode is about my project titled “Methanol Production via CO2 Hydrogenation on Promoted Cu-based Catalyst”. This project was carried out and submitted for Bachelor degree requirement in Petroleum Engineering from ……………..to ………………
[CE 1.1]
The project was done during the graduate course in Petroleum Engineering in ____________________. The project was executed all alone by me under the supervision and advantageous guidance of Dr. Pradip Chnadra Mandal.
[CE 1.2]
My first career episode is related to a project which I did when I was in ______ year of engineering studies. I have studied the Hydrogenation in my _______ year in details. Since then the topic has intrigued me to work in this segment on a broader scale. This project was based on the Methanol Production via CO2,, Hydrogenation on Promoted Cu-based Catalyst. I have chosen this project in consent with my Project Supervisor. I worked closely for this project and got the desired results likewise. My project was completed well before deadline and was acknowledged in a respective manner by the Project Supervisor & Dean of the Petroleum Engineering Department.
A number of studies on methanol production have been reported over copper Cu-based catalysts on Alumina Support (Cu/ZnO/Al2O3). This catalyst undergoes a deactivation process due to the water produced and the heat released from the reaction. This project focused primarily on synthesizing the copper based catalyst on the Carbon Nanotubes (CNTs) as catalyst support for CO2 hydrogenation to synthesize methanol.
[CE 1.3]
The purpose of starting this project was to fulfil a major milestone, dissertation, in partial fulfilment of the requirements for a Bachelor of Engineering (Hons). I worked under direct supervision of Dr. Pradip Chandra Mandal, Lecturer in the Department of Chemical Engineering with consent to the Department of Petroleum Engineering at Universiti Teknologi PETRONAS.
This project focused on the development of the methanol synthesis catalyst to find a promising catalyst that increases the methanol selectivity and long life activity.
I primarily focused on the effect of adding promoters such as niobium (Nb) and zirconium (Zr) on the properties of the catalyst. Besides that, the job included characterizing the physical and chemical properties of the catalyst using some characterization techniques such as Scanning Electron Microscopy (SEM), EDX and TPR. The catalytic performance of the synthesized catalysts was carried out on a high pressure reactor analysis system made up of micro-activity fixed-bed reactor with an on-line gas chromatograph with flow ratio of H2/CO2 is 3:1 under operating temperature and pressure of 250℃ and 2.25 MPa respectively.
[CE 1.4]
This project work was completed by me alone. The Hierarchy of the project is displayed below:
[CE 1.5]
The project was completed in flock with a team member and my errands were:
[CE 1.6]
I did the Project management of the system which involved the modularization of the project in to definite number of stages & created a classification of the activities to be accomplished. I prearranged the entire project in two stages.
PHASE 1: During this phase I finalized the study of the conceptual as well as philosophical nitty-gritties of the proposed work & drew up an approach to achieve the desired goal.
PHASE 2: In this phase; I have employed strategies for successful execution. After execution I carried out all the necessary proceedings of the project.
[CE 1.7]
My Roles & Responsibilities were -
[CE 1.8]
Problem A
I faced the issue of water presence as a by-product in methanol production via CO2 hydrogenation process. Some amount of water was adsorbed by alumina supported copper catalyst and this has deactivated the catalyst. Furthermore, Cu particles have undergone sintering process due to the exothermic reaction.
Solution
To overcome this issue, I used the carbon nanotubes (CNTs) and found that it does not adsorb H2O and has very good thermal conductivity. These properties make CNTs the most suitable and highly dispersed catalyst for CO2 hydrogenation process.
[CE 1.9]
Problem B
Another issue occurred during the reaction process when determining the most optimal temperature and pressure for the reaction to take place inside the reactor.
Solution
After several trial-and-error attempts, I found that the most optimal operating conditions for the reaction in the tube of the micro-activity fixed-bed reactor under temperature and pressure of 250 oC and 1 bar respectively. Generally, the reaction of the methanol production from synthesis gas is conducted for 8 hours. The reactor lines, tubes and valves were heated up to 180 oC to prevent product condensation process.
[CE 1.10]
After the synthesis of the catalyst, I designed a suitable reactor for the reaction of CO2 hydrogenation to produce methanol to evaluate the performance of the synthesized Cu-ZnO catalysts.
The CO2 hydrogenation reaction takes place in a micro-activity fixed-bed reactor. My design was based on some calculations to determine the dimensions of the reactor. I found that outer diameter = 14.5 mm and inner diameter = 9 mm and the length of the tube = 305 mm with total volume of 20 ml. The catalytic performance test was conducted under the same operating condition for all the prepared catalysts in order to have reasonable comparison for all catalysts.
I started the reaction with holding 0.1 g of the prepared catalyst in the tube of the micro-activity fixed-bed reactor between two layers of quartz wool. Then, the tube filled with the catalyst was placed inside the furnace of the reactor. The door of the reactor was closed and helium gas was purged to the reactor at flow rate of 15 ml/min for period of 30 minutes for removing the existing air and contaminants out of the tubes of reactor and gas chromatography. The reduction of the catalyst was in 5 vol% H2 in helium gas at 20 ml/min for 2 hours under temperature and pressure of 250 oC and 1 bar respectively.
[CE 1.11]
I did a handful of calculations to prepare the CNT supported Cu/ZnO based catalyst (15wt%Cu-ZnO /84wt% CNT). The calculations involved the amount of materials used in the project as well as the preparation of the solutions. The following are some calculations for synthesizing 1 g of catalyst. Weight of metals (Cu/ZnO) (g) is calculated by the following equation: weight of metal (g)= total weight of the catalyst × percentage of desired metal loading weight of metal (g)=1 g ×15⁄100 = 0.15 g. Weight of Cu (g)=0.15×70⁄100 =0.105 g. Weight of ZnO (g) =0.15×30⁄100= 0.045 g. The weight of precursors: weight of 〖(Cu(〖NO〗_3)〗_2.3H_2 O) (g)=(0.105 g Cu ×241.6 g/mol 〖Cu(〖NO〗_3)〗_2.3H_2 O)/(63.5 g/mol Cu)=0.3995 g weight of 〖(Zn(〖NO〗_3)〗_2.3H_2 O) (g)=(0.045 g ZnO ×297.48 g/mol 〖Zn(〖NO〗_3)〗_2.3H_2 O)/(81.41 g ZnO)=0.1644 g Weight of support (CNT): weight of support (g)=total weight of the catalyst -weight of metal weight of Alumina (g)=1.0 -0.15=0.85 g Preparation of precursor solution (0.5M): number of moles of 〖(Cu(〖NO〗_3)〗_2.3H_2 O) =Molarity ×volume number of moles of 〖(Cu(〖NO〗_3)〗_2.3H_2 O) = Mass/(Molar Mass) = (0.3995 g)/(241.6 g/mol)=0.00165 mol number of moles of 〖(Zn(〖NO〗_3)〗_2.3H_2 O) = Mass/(Molar Mass)= (0.1644 g)/(297.48 g/mol) =0.000553 mol Total number of moles = 0.00165+0.000553 = 0.0022 mol Volume = (Number of moles)/Molarity= 0.0022/0.5=0.0044 L=4.4 ml H_2 O
[CE 1.12]
I was accountable for underpinning feasibility study which included technical & economic consideration of this project. I calculated the whole project cost scrutiny and presented it to my project supervisor for prior approval.
[CE 1.13]
During this Project I gained extensive experience & knowledge of process simulation, debottlenecking & troubleshooting of different equipment. I also ameliorated my decision making & leadership skills. The project was efficaciously premeditated & I thoroughly verified the employability of project. This project elevated my project management, planning, analysis and implementation skills. The project was successfully completed and was highly admired by the Project Supervisor & head of the petroleum engineering department.
We hold the apex position in providing services regarding CDR writing for engineers Australia. We are known to have very high success records for consistent team of professional writers having years of experience in the field of CDR preparation. We provide the best and trusted service for CDR writing and reviewing of all kinds of engineering disciplines. We provide services for career episode writing, plagiarism check and removal etc.
Should you need any further information, please do not hesitate to contact us.
Contact: +61-4-8885-8110
WhatsApp: +61-4-8885-8110
(Australia, USA, UK, UAE, Singapore, New Zealand)